No products in the cart.
Peptides in Joint Tissue Research and Regenerative Implications
Awd erJoint‑Peptide Frontiers
In recent years, peptides have emerged as a versatile class of bioactive molecules in musculoskeletal research. Within the context of articulating joints (i.e., the complex structures that allow movement between skeletal elements), peptides are being investigated for their potential to modulate the extracellular matrix, cellular behavior, and tissue engineering strategies.
This article presents a speculative yet data‑grounded overview of “joint peptides” (i.e., peptides relevant to joint tissues such as cartilage, synovial interface, or osteochondral regions), their possible uses in research domains, and illustrative examples of how they might be applied. Emphasis is placed on tissue‑engineering, biomaterial functionalization, and mechanistic explorations.
Peptide Functions in Joint‑Related Tissue Contexts
Studies suggest that peptides relevant to joint tissues may assume multiple roles: they might act as functional ligands, scaffold‑modifiers, matrix mimics, or signaling modulators. Some key conceptual roles might include:
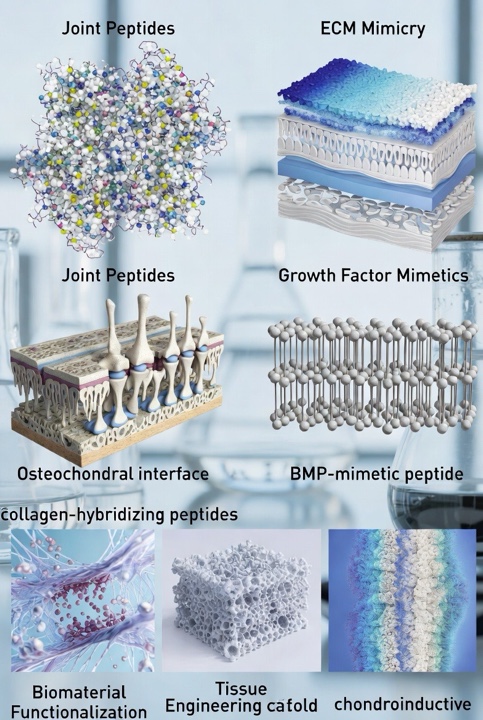
- Extracellular matrix (ECM) mimicry – Peptides derived from or inspired by ECM proteins (such as collagen, aggrecan, or link‑protein motifs) are believed to be employed to mimic aspects of the cartilage matrix. Such peptides are thought to support cell–matrix interactions, promote adhesion, or alter differentiation trajectories of progenitor cells.
- Signaling modulation – Studies suggest that peptides may be engineered to approximate the activity of growth factors (for example, mimetic peptides of TGF‑β or BMP family members). These seem to engage specific receptor pathways in chondrocytes or progenitor cells, thereby supporting cartilage or osteochondral tissue formation.
- Functionalization of biomaterials – Research indicates that in tissue‑engineering scaffolds, peptides are increasingly used to functionalize surfaces (e.g., hydrogels, nanofibres) to provide specific binding, cell recruitment, or differentiation cues. In joint tissue research, such peptide‑modified scaffolds are thought to help mimic the complex micro‑environment of cartilage or the osteochondral interface.
- Matrix degradation or remodeling probes – Investigations purport that some peptides may be utilized as probes to detect or interact with degraded ECM components in joint tissues (for example, collagen-hybridizing peptides that bind unfolded collagen chains). Such peptides have been hypothesized to support mechanistic investigations into cartilage degeneration or repair.
Research Domains and Implications
The relevance of joint‑relevant peptides is speculated to span several research domains:
- Cartilage and osteochondral interface engineering research
A major challenge in joint research is the regeneration of hyaline cartilage and the osteochondral interface (the transition zone between cartilage and underlying bone). Investigations purport that peptides may be used in the following ways:
- Exposure of research models to chondroinductive peptides — For instance, a recent review outlines that peptides engineered to induce chondrogenesis (so‑called “chondroinductive peptides”) may promote cartilage repair by binding to growth factor cytokines or ECM components.
- Scaffold functionalization — Peptide‑based biomaterials have been reported to assist in cartilage and bone regeneration by virtue of peptides that regulate cell adhesion, differentiation, and matrix formation.
- Example: The BMP‑mimetic peptide (“BMP peptide” sequence KIPKASSVPTELSAISTLYL) has been reported to stimulate glycosaminoglycan production and cartilage‑matrix accumulation without inducing hypertrophic markers in human mesenchymal stem cells.
These implications suggest that peptides may serve as directed cues within engineered constructs to promote cartilage‑specific outcomes in joint research models.
- Mechanistic study of joint ECM degradation/remodeling
In research exploring degenerative joint conditions (such as cartilage breakdown), peptides have been theorized to play roles:
- Collagen-hybridizing peptides (CHPs) are synthetic peptides believed to bind to denatured collagen strands by forming a triple‑helix hybrid with the unfolded collagen. These have been relevant as probes of collagen disruption in tissues.
- In the context of cartilage research, peptides that target matrix metalloproteinase (MMP)‑sensitive sequences or collagen cleavage fragments may help to map degradation pathways or evaluate interventions.
Thus, studies suggest that peptides may serve as molecular tools in joint tissue research, enabling fine‑grained investigation of matrix remodeling, cell–matrix interactions, and mechanobiology.
- Biomaterial and research‑device development
Beyond purely biological investigations, peptides are thought to be leveraged in biomaterial design for mammalian joint tissue:
- Hydrogels or nanofibrous scaffolds functionalized with chondro‑adhesive peptide motifs or growth‑factor‑mimetic peptides have been developed to simulate the cartilage micro‑environment.
- For instance, researchers have incorporated peptides derived from collagen (such as GFOGER, P15) into scaffolds to possibly enhance cell adhesion or matrix deposition in osteochondral defect models.
These mechanistic tools are speculated to advance joint tissue engineering research, providing platforms for cell seeding, mechanical loading studies, or disease modeling research.
Examples of Joint‑Relevant Peptides
Here are several illustrative peptides along with their characterized roles in joint‑tissue research:
- BMP‑mimetic peptide (KIPKASSVPTELSAISTLYL): As noted above, this peptide was ascertained to promote chondrogenic differentiation of human MSCs by increasing glycosaminoglycan and collagen production, without excessive hypertrophy.
- Collagen‑mimetic or derived peptides (e.g., GFOGER, P15): These peptides have been hypothesized to mimic collagen binding domains and have been used in scaffolds to promote integrin‑mediated adhesion of progenitor cells or chondrogenic cells; their use in joint‑tissue scaffold design is documented.
- TGF‑β mimetic peptides (e.g., cytomodulins “CM” peptides): Small oligopeptides derived from TGF‑β signaling domains (sometimes immobilized) that may promote chondrogenic differentiation when anchored to biomaterial surfaces.
Concluding Remarks
In summary, peptides have been hypothesized to hold considerable promise in joint‑tissue research. From ECM‑mimetic scaffolding motifs and growth‑factor‑mimetic sequences to mechanistic probes of matrix degradation and functionalized biomaterials for cartilage regeneration, peptides are versatile tools. Their modularity, ease of synthesis, and tunability make them attractive for research domains spanning cartilage engineering, osteochondral interface modeling, matrix remodeling investigations, and mechanobiology. Researchers are encouraged to click here to learn more about the potential of peptides.
References
[i] Li, Y., Ramshaw, J. A. M., Werkmeister, J. A., Melrose, J., & Schaffer, J. (2015). Collagen‑mimetic peptide‑modifiable hydrogels for articular cartilage regeneration. Biomaterials, 54, 213‑225. https://doi.org/10.1016/j.biomaterials.2015.02.079
[ii] Luke, E. N., Ratnatilaka Na Bhuket, P., & Yu, S. M., Weiss, J. A. (2023). Targeting damaged collagen for intra‑articular delivery of therapeutics using collagen hybridizing peptides. Journal of Orthopaedic Research, 41(11), 2424‑2432. https://doi.org/10.1002/jor.25577
[iii] Parmar, P. A., Chow, L. W., St‑Pierre, J.‑P., Horejs, C.‑M., Peng, Y. Y., Werkmeister, J. A., & Stevens, M. M. (2015). Collagen‑mimetic peptide‑modifiable hydrogels for articular cartilage regeneration. Biomaterials, 54, 213‑225. https://doi.org/10.1016/j.biomaterials.2015.02.079
[iv] Zarei, M., & Rezvani, M. (2023). Multipotential role of growth factor mimetic peptides for osteochondral tissue engineering. International Journal of Molecular Sciences, 23(13), 7388. https://doi.org/10.3390/ijms23137388
[v] Yang, Z., et al. (2023). Targeting damaged collagen for intra‑articular delivery of therapeutics using collagen hybridizing peptides. (This is essentially the same as #2 but gives additional mechanistic data.) J Orthop Res. 41(11), 2424‑2432. https://doi.org/10.1002/jor.25577















Leave a Reply